The Cosmic Ice Laboratory - Research
The Cosmic Ice Laboratory - Our Research
Our research group is part of the Astrochemistry Laboratory in the Solar System Exploration Division at NASA's Goddard Space Flight Center, and is a member of the Goddard Center for Astrobiology. We specialize in studying the spectra, the chemistry, and the physical properties of ices relevant to comets, icy satellites and planets, and the coatings of dust grains in the interstellar medium. Although many cosmic ices are dominated by H2O, they also contain "prebiotic" molecules such as CO, CO2, CH4, NH3, and CH3OH. In studying these molecules we are probing the early, ancient chemistry which eventually led to the origin of life.
In our laboratory we prepare ices by using a cryostat to condense gas-phase mixtures to temperatures as low as 10 K. The ices are made in a vacuum system to simulate the low pressure of outer space, and also to avoid undesirable contamination from the Earth's atmosphere. An infrared spectrometer is used to record spectra of an ice during experiments.
Our laboratory set-up is unusual because it is interfaced not only to a Van de Graaff accelerator, that can produce protons at energies up to about 1 million electron volts (1 MeV), but also to a hydrogen-discharge lamp that supplies ultraviolet (UV) photons (energy ~10 eV). The high-energy protons simulate the magnetospheric or cosmic ray radiation exposure expected for planetary, cometary, and interstellar ices, while the UV photons from our lamp simulate the Solar or interstellar UV field. When either the protons or UV photons strike an ice sample they produce ionizations and excitations, resulting in chemical reactions to make new molecules. By comparing infrared spectra taken before and after this processing of an ice, we can identify molecules formed by either radiolysis or photolysis.

Comet Hyakutake

The Eagle Nebula is an interstellar
cloud of gas and dust.
Ices in interstellar environments often are accompanied by dust grains. With comets, both dust grains and gases subliming from ices are observed. In the Cosmic Ice Laboratory we use silicatematerials to simulate cometary, and other, grains. Ices formed on thesesilicates are used to investigate ice-grain interactions.
When warmed to room temperature, many irradiated icy mixtures leave an organic residue, which can be analyzed by chromatography and mass spectrometry. We used these methods to show that hexamethylenetetramine (HMT) is synthesized when H2O + CO + NH3 + CH3OH mixtures are processed with either photons or protons. HMT is known to produce amino acids if hydrolyzed.
Results from our experiments explain and predict the existence of specific molecules on space objects. For example, since SO3 is formed in laboratory irradiations of SO2 ice then SO3 is also expected on the Jovian satellite Io, whose surface SO2 experiences intense radiation from Jupiter. Our experiments on H2O + CH3OH mixtures suggested ethylene glycol as an interstellar molecule, and indeed this molecule was later detected in interstellar space. In other work we showed that the oft-debated "XCN" infrared feature in interstellar ices is due to the cyanate ion (OCN-), and that the anomalous HCN-HNC ratio in comets can arise from radiation exposure to cometary molecules. Still other experiments have concerned the formation of H2O2 on Europa, an icy Jovian satellite, and the synthesis of C2H6 and C3O2 in comets.
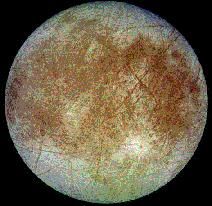
Europa, a moon of Jupiter, has ices such as H2O, H2O2, CO2, and SO2 on its surface.
