Early Career Scientist Spotlight
Dr. Yukiko Yarnall (she/her/hers)
Laboratory Astrochemist
Astrochemistry Laboratory (691)
What is your research focus?
My postdoctoral research is strongly focused on ices and salts in space, specifically to help Goddard remain an eminent repository for infrared (IR) spectra of laboratory-created ices for space- and ground-based observations, spacecraft, planetary rovers, and even for sample-return missions. Ices are present in space, perhaps most famously as icy-bodied comets. We’ve learned from NASA missions that ices are also present as aerosols in the atmosphere of the organic-rich moon Titan and on dust grains in the molecular clouds that are the birthplace for stars. Interstellar ices provide a place for efficient chemical reactions to occur, increasing the chance of a collision between molecules and atoms and for the absorption of the energy from the heat of reaction. Many molecules have been identified in a solid phase that would facilitate the formation of prebiotic molecules.
My primary science question is 'Are there other molecules outside Earth still unidentified?' Spectral analysis is one method we use to investigate conditions in space and on extraterrestrial bodies and for the identification and quantification of observed matter (in our case, ices). Low- temperature laboratory spectroscopy is used to measure physical and optical properties of interstellar ice analogs, and our data provide a means to interpret observational spectra obtained from telescopes and spacecraft and laboratory spectra obtained experimentally. Our group prepares interstellar and solar-system ice analogs by vapor deposition onto a cold substrate in a stainless-steel vacuum chamber and then measures the mid- and near-infrared (IR) spectra of the sample. We use a quartz-crystal microbalance, a mass spectrometer, laser interferometers, and Fourier transform spectrometers for determining mass densities, refractive indices, IR intensities (apparent absorption coefficients and apparent band strengths), vapor pressures, energies of sublimation, and optical constants. We catalog and share our data to assist with the most-accurate spectral analysis of observational results, as well as for experimental and theoretical studies (including modeling) to understand chemical evolutions in space.
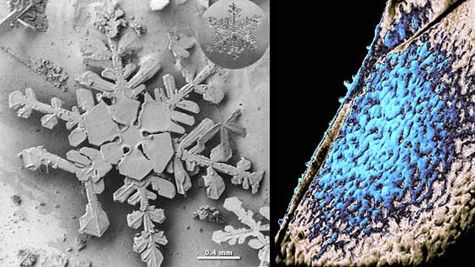
Credits: Left: NASA/Earth Observatory; Right: NASA/ARC/P. Jenniskens and D.F. Blake
What do you enjoy the most about your job?
I LOVE experimentation. During the lengthy time it takes to get a single result in the lab, I enjoy imagining better ways of obtaining reliable data while I’m preparing ice samples. Our ‘space’ ice preparation steps are some of the most important procedures we have for a proper experiment. However, we sometimes fail to get useful results. Some molecules cannot produce the well-defined interference fringes needed for the determination of ice thickness. Other times, amorphous samples crystallize, ruining many hours of time invested in an experiment. In these cases, slightly modifying our preparation conditions to improve them becomes important and sometimes works! Of course, I have frequent disappointments but, at the same time, I am satisfied that we never quit and we always charge ahead. So, I really enjoy the continuous process of thinking of new ways to get better outcomes.
I am also fond of GOOD teamwork. I absolutely enjoy my daily discussions with colleagues about producing quality solutions. As a team, we stay up to date on both current and future research worldwide, and I’ll admit, we enjoy eating sweets and good homemade food together at times.

Credit: Reggie Hudson and Perry Gerakines
Did you always know that you wanted to be an Astrochemist?
No, but as a child I loved a cartoon called ‘Space Battleship Yamato’ (a.k.a., ‘Starblazers’ in America), and I had never given any thought to studying space, let alone conducting research at NASA – that would’ve been pure fantasy at the time. I was curious as a child. During high school in Japan, I originally thought I’d be a pharmacist, but I ended-up as a nutritionist. While both careers were centered around chemistry, becoming a chemist wasn’t an option until going back to school later in life as an undergraduate here in the U.S. Now, I am an astrochemist who is curious about ice in space. Life can be interesting and unpredictable.

Credit: Reggie Hudson
What is one of your favorite moments in your career so far?
My favorite GSFC career moment was when my NASA Postdoctoral Program (NPP) supervisor greeted me with “Konnichiwa”, or “hello” in Japanese, during our first meeting in the Goddard waiting room. We then went to the Cosmic Ice Lab to meet my new teammates, whose names I’d seen in publications, so I was very nervous! Thankfully, other colleagues also spoke to me in Japanese making me comfortable and feeling very welcome. I will never forget that moment.
I also love teaching, so my favorite academic moment was when I taught general chemistry to students in the ‘Enlisted to Medical Degree Preparatory Program’ (EMDP2) who were preparing to apply to medical school. Most of these service members had families, but still studied very hard. As a military wife, I understood them, and returning to the academic world as an adult was a struggle, so I was honored for the privilege of teaching them and thanked them for their service.

Credit: Uniformed Services University of the Health Sciences (USUHS)
What early career advice do you have for those looking to do what you do?
I started my most recent school study in Hawaii in 2007 with English as a second language (ESL) class so that I could earn a US high school diploma, and I eventually ended with a PhD. I was raising three young boys and had to move while my husband deployed overseas for a year as a reservist, more than twice, for which I am proud of him. Many times, I’ve felt lonely or isolated in school because of language and cultural issues, but also because I was older than the other students. I was lucky that I loved to learn new things, meet wonderful peers, and it helped that I had nice professors. In 2009, during my undergraduate study, I didn’t know much about the space field, but I was inspired by astrochemistry and atmospheric chemistry by my former professor and PhD adviser - a former postdoc with the Cosmic Ice Laboratory. I landed a graduate research fellowship and a graduate research overseas internship through the National Science Foundation. I read many Cosmic Ice Lab publications for my graduate research, but never thought that I would work here. However, after contacting my current adviser, showing him my studies, and informing him that I wanted to apply to the NPP: “the rest is history”, as we say. So, my early career advice is straight-forward:
- Follow your passion wherever it takes you and never, ever give up.
- Everyone needs some help or guidance eventually. So, if you need help, don’t be afraid to ask, and be persistent about it.
What are your future research interests and goals?
I would like to analyze actual ices and salts from sample-return missions, especially cometary ices and salts. Comets may have triggered life to emerge on Earth during the heavy bombardment era. Ammonium cyanide, a precursor of amino acids and nucleobases (e.g., Oro 1960), can be formed in a comet’s nucleus and has been studied in our Cosmic Ice Lab (Gerakines et al., 2004). I simply cannot wait for the sample-return from the nucleus of a comet in the future.
I’m also interested in spreading the knowledge of space science to a wide audience. After my research career, if I were a teacher, I would pass the splendor of science to young people who might want to become scientists and to the elder generation to stimulate their minds.
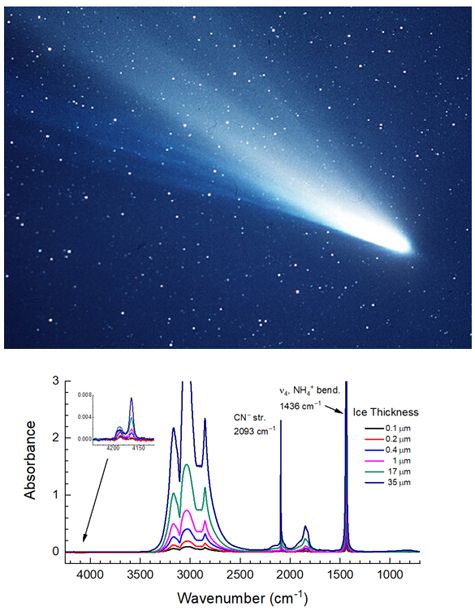
Credits: Top: NASA; Bottom: Yukiko Yarnall
What do you like to do in your free time?
Mainly, I am crazy for tennis and can usually be found on the court. I played when I was young and again when I visited Hokkaido University as a 2017 Graduate Research Opportunities Worldwide fellow (http://www.lowtem.hokudai.ac.jp/astro/index_e.html). Today, I am the ‘designated’ watermelon person every weekend sharing small picnics and peaceful moments with my tennis friends while waiting for our turn to play. I also still love watching cartoons, reading manga, and browsing antique stores and thrift shops.

Credit: Anh Vu Ly
Biography
Home Town:
Kanagawa, Japan
Undergraduate Degree:
BS in Chemistry and BS in Mathematics, George Mason University, Fairfax, Virginia
Post-graduate Degrees:
MS in Mathematics, George Mason University, Fairfax, Virginia
PhD in Chemistry and Biochemistry, George Mason University, Fairfax, Virginia
Link to Dr. Yarnall's GSFC Bio
